What Gets Measured Gets Done
We set standards and benchmark the performance of pharmaceutical companies, assessing how new medicines and vaccines are researched, marketed, and made accessible to patients.

We set standards and benchmark the performance of pharmaceutical companies, assessing how new medicines and vaccines are researched, marketed, and made accessible to patients.
Why go to the trouble of ranking companies on their social responsibility? While there are many determinants of health, access to medicines and vaccines is a critical factor. And, when we think about access, we have to think about the role of the industry, as it sponsors the majority of clinical research supporting new medicines, vaccines and device approvals, disseminates drug safety and efficacy information, sets prices and much more. Before Bioethics International, no one organization consistently reviewed pharmaceutical companies on their bioethics to ensure high performance on ethics and legal requirements.
We help ensure trial participants’ involvement is not wasted; that we advance scientific knowledge and accessible products for patients. To date, we’ve performed transparency, accessibility, equity, and human rights due diligence on thousands of trials, enrolling over 1 million patients and participants around the world.
We make sure that the medical evidence for new drugs is publicly available, so doctors can prescribe the right drug for the right patient. This affects a large proportion of the US population, as in the past 30 days:
49% of people have used at least one prescription drug

On average, people age 45 and older say they take 4 prescription medications daily

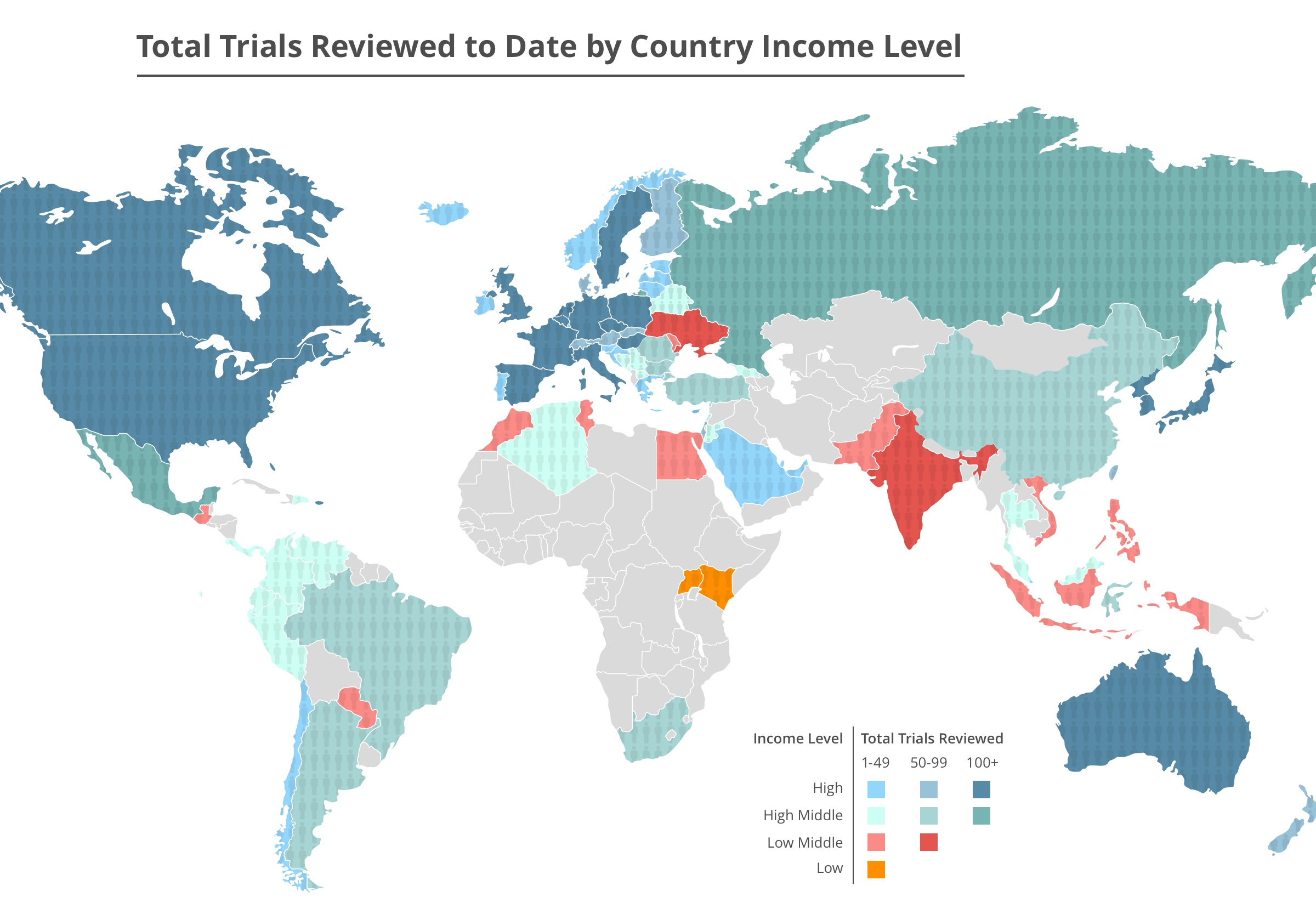
When we reviewed medicines and vaccines for HIV, TB, breast cancer, rare diseases, pediatric meningitis, and many other conditions, approved by the FDA in 2012, we noticed there was room for improved transparency. We found:
35% of trial results per drug were publicly unavailable

1/2of all drugs had undisclosed results for their phase 2 or 3 trials
20%of large companies (2 in 10) disclosed all trial results
![]()
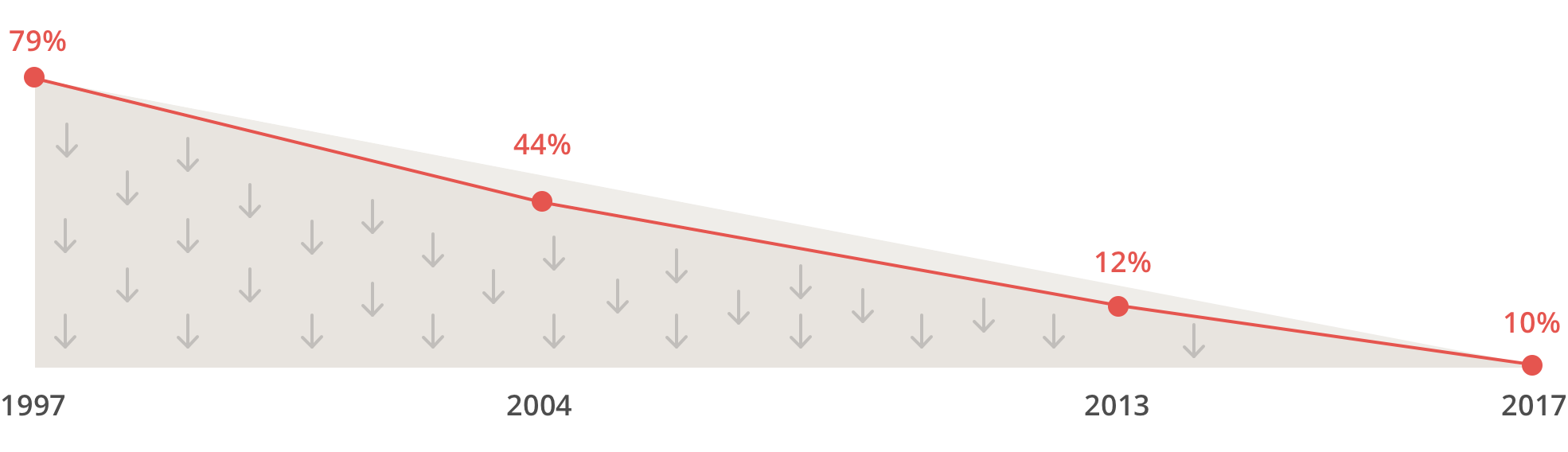
We realized low transparency may be contributing to the lack of trust in pharma companies and that distrust can create barriers for patients to participate in trials and take their medicines and vaccines. The percentage of Americans who trust that pharma companies are honest & ethical has been decreasing, year by year:
That’s why we published the First Good Pharma Scorecard in 2015— to focus on the ethical issues impacting patients the most and to rebuild patient-centricity and trustworthiness in healthcare innovation. Our 2nd Good Pharma Scorecard, published in 2017 benchmarking drugs approved by the FDA in 2014, already shows meaningful progress has been made:
The proportion of new drugs with undisclosed phase 2 or 3 trials has gone down from
2015 50%

2017 30%

The public availability of results for trials conducted in patients, for each drug, went up from
2015 87%

2017 96%

The overall industry scores went up from a median of
2015 88%

2017 91%