Good Pharma Scorecard
We rank companies on ethics and social responsibility performance criteria, focusing on issues that are critical to patients and public health.
The Good Pharma Scorecard ranks companies on their bioethics and social responsibility performance and governance, taking into account ethics and legal standards. Measures evaluate the integrity of clinical trial designs, the accessibility of medicines, marketing practices, diversity and fair inclusion, among other ethics and human rights issues. Below is a sample of our company and product rankings.
Transparency and data-sharing practices
Clinical trial design integrity
Diversity, equity and fair inclusion in research and the workplace
Accessibility of medicines, vaccines and devices
Fair benefit-sharing
Marketing practices
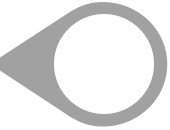
Median: 81%

Company ranking
higher than last ranking

Company ranking
lower than last ranking
Company ranking
unchanged from last ranking